As seen in






What we do
Atropos Health creates high-quality personalized Real-World Evidence in minutes and at scale, creating the trusted currency of value in healthcare.
Atropos Health is the industry leader in producing novel, methodologically credible RWE-reports with unprecedented quality and speed, fueled by our mission to improve healthcare outcomes for everyone.
Healthcare data is now plentiful, if siloed, but converting it to high-quality evidence takes months and is resource intensive, limiting it to expensive research settings.
Our Results in Numbers
100%
Success rate in publishing studies in peer-reviewed journals. Over 50 publications including Cell, NEJM Catalyst and more.
10K+
Clinical questions converted into studies run on behalf of our clients. Publication grade observational studies can be re-run on your own data to make policy changes or practice precision medicine.
$100M
Saved by our clients in expedited analytics, maximized RWD assets, medication cost savings, patient throughput and care management programs.
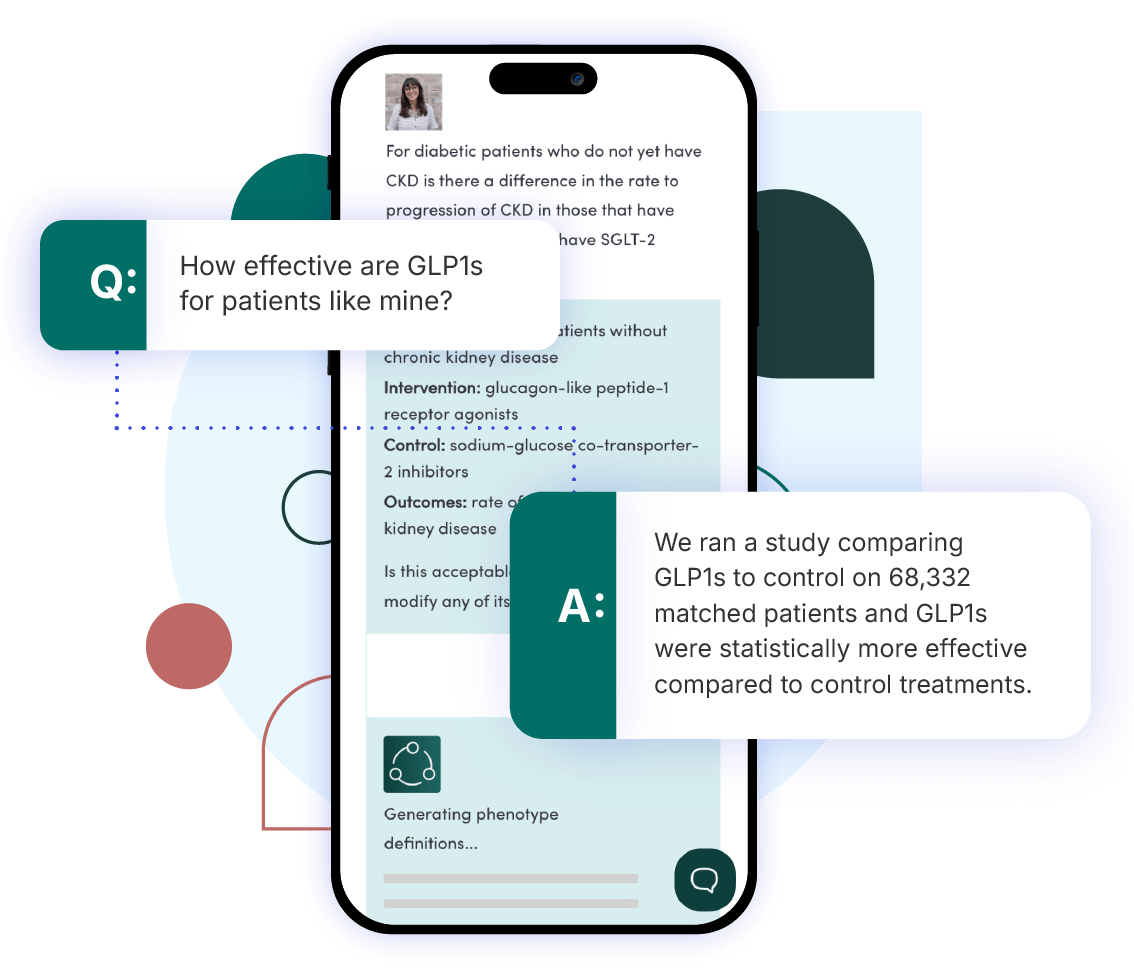
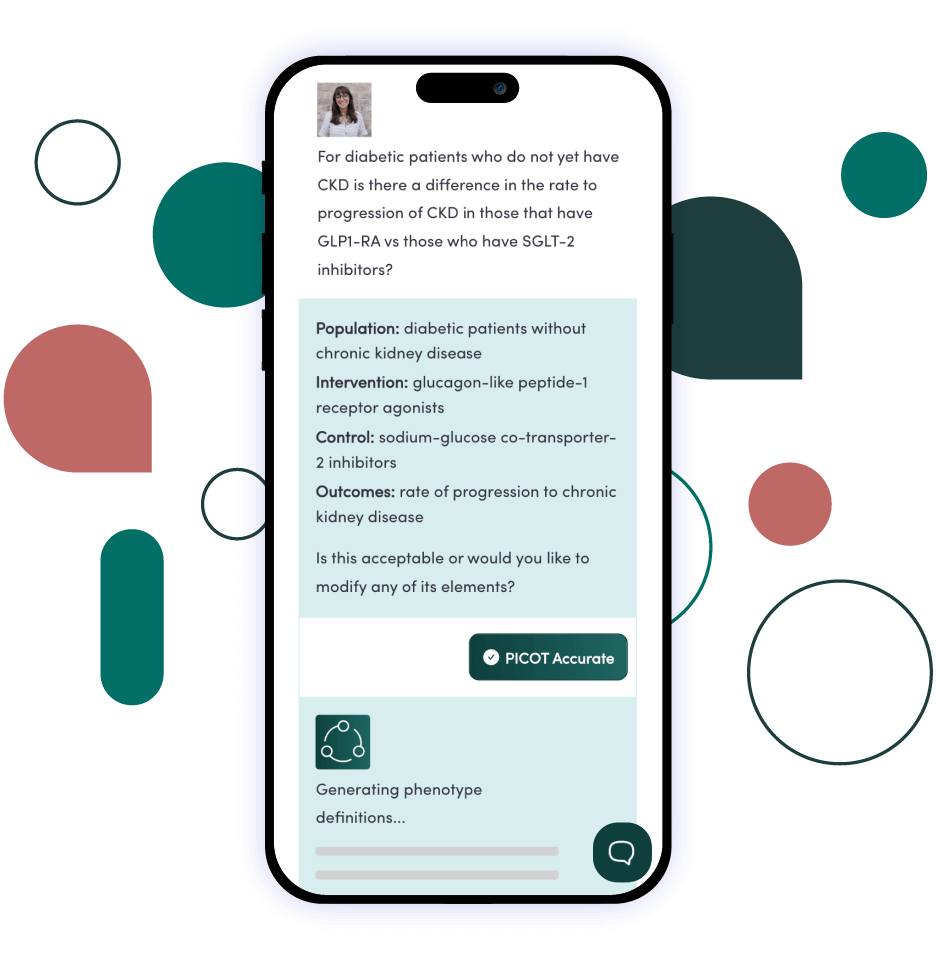
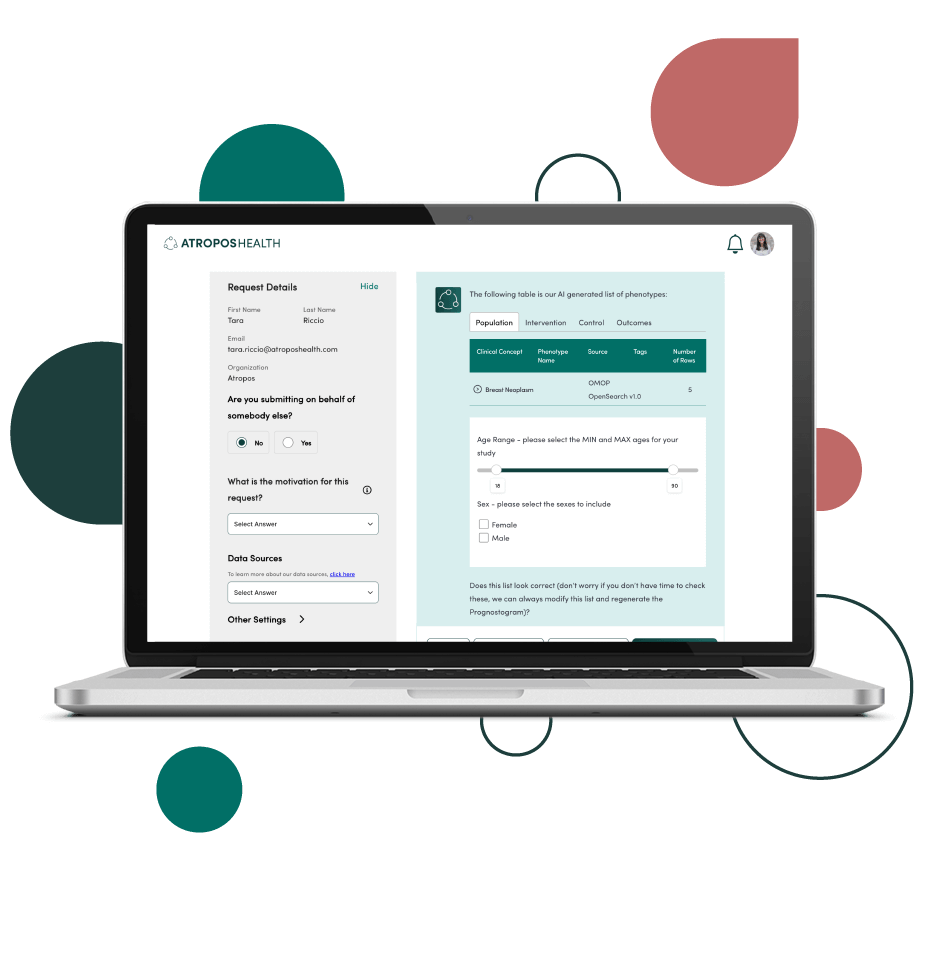
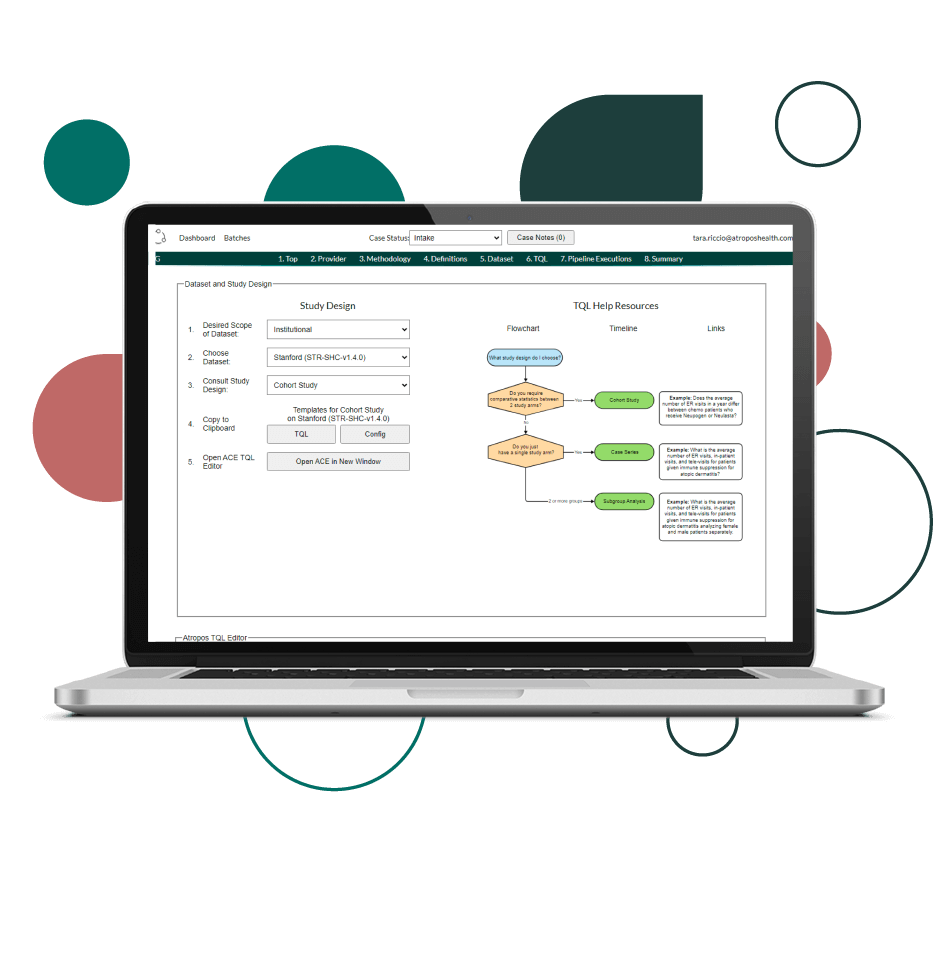
Find the answers you need with our unique combination of technology, data, and tools.
Our innovative technology is cloud-based, scalable, and provides high-quality rapid analytics. We ensure the data used to answer your clinical question is fit for purpose. We provide on-demand data access with novel data strategy and fitness scoring. Our accelerated tools grant your users the access they need to publish in academic journals, perform queries to test regulatory grade concepts, and select the phenotypes for a particular use case.
Testimonials

“We were able to utilize Atropos Health to evaluate medications for formulary consideration. Real World Evidence (RWE ) enabled us to understand patient outcomes on these therapies and resulted in removal...


Resources
Check out our latest press releases, news and media coverage, videos and blog
Transparency, along with accuracy, quality and speed are what drive us. Read about our methods, results and technology to see how we can partner to improve the use of RWE in healthcare today.
Fierce Healthcare Names Atropos Health a “Fierce 15” Company of 2026
Fierce Healthcare has named Atropos Health as one of 2026’s “Fierce 15” healthcare companies. The annual special report features...
Atropos Health Chief Medical Officer and Co-founder Dr. Saurabh Gombar Speaks with Barbara Pazur of CNET
In a recent CNET feature exploring the launch of OpenAI's dedicated ChatGPT Health tab, Atropos Health Chief Medical Officer and...
We are a mission driven organization
Atropos Health represents more than a decade of technology, research, and clinical progress.
We’re building the future of evidence-based medicine. It’s time for every clinical decision and research inquiry to be backed by personalized evidence.
The promise of learning from the care of past patients is a concept that has been discussed for a generation now. Atropos Health uses a combination of technology, data, and a physician-centered service to fulfill the promise of data-driven medicine for individual patients.
Do You Want to Build the Future of Evidence-Based Medicine?
Atropos Health might be right for you.

Atropos Health in the news
Atropos Health Chief Medical Officer and Co-founder Dr. Saurabh Gombar Speaks with Barbara Pazur of CNET
In a recent CNET feature exploring the launch of OpenAI's dedicated ChatGPT Health tab, Atropos Health Chief Medical Officer and...
Atropos Health CEO and Co-founder Dr. Brigham Hyde Speaks with Brian Gormley of The Wall Street Journal
In a recent Wall Street Journal feature discussing the entry of OpenAI and Anthropic into the digital health sector, Atropos...
Atropos Health Named to the 2026 New York Digital Health 100
Atropos Health has been named to the 2026 New York Digital Health 100 (DH100), an annual recognition honoring the most...
Contact us today
Learn how you can scale your capabilities to learn from and act on healthcare data.
- Danner M, Debrouwere M, Rummer A, Wehkamp K, Rüffer JU, Geiger F, Wolff R, Weik K, Scheibler F. A scattered landscape: assessment of the evidence base for 71 patient decision aids developed in a hospital setting. BMC Med Inform Decis Mak. 2022 Feb 17;22(1):44. doi: 10.1186/s12911-022-01777-x. PMID: 35177043; PMCID: PMC8855583.