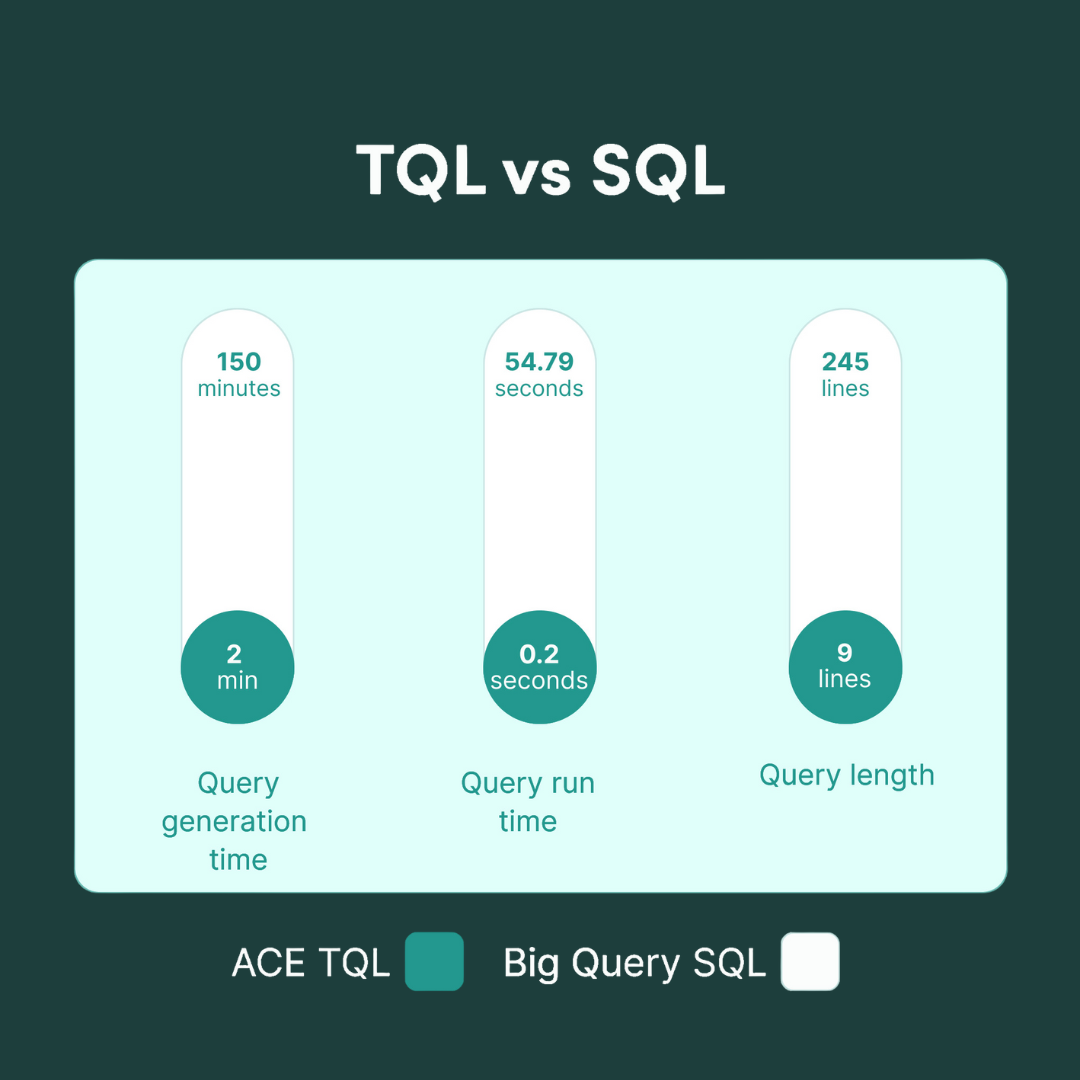
Data science teams achieve faster results across multiple therapuetic areas (TAs)
Atropos Health combines rapid cohort creation with automated, state of the art data science.
Generate the insights you need with short deadlines and longer projects
Examples of previous use cases
Trial design and optimization
– Trial emulation
– Inclusion exclusion criteria evaluation
Epidemiology and RWE
– Market sizing
– Unmet need
– Protocol development
– Regulatory support and synthetic control arms
Business development and licensing
– Market opportunity
– Patient journey
– Unmet need
Our Results in Numbers
5-10X
Study and insight output in 10% of the time.
$21M
ROI savings from reduced outsourced service and data costs.
48hr
SLA on Real-World Evidence studies.
Testimonials
"Atropos reduced dependence on other tools that are more hands on. Atropos can operate more independently."
Pharma Client
VP of Data Science
"Atropos has the competitive advantage when it comes to speed."
Pharma Client
Medical Executive
The Atropos Evidence™ Network has the Real-World Data (RWD) available on day 1 to answer your questions
Learn more about the Atropos Evidence™ Network.

