Cell Publishes “A widely distributed gene cluster compensates for uricase loss in hominids”
On August 3rd 2023, Cell published “A widely distributed gene cluster compensates for uricase loss in hominids” by Yuanyuan Liu 1, J. Bryce Jarman 1, Yen S. Low 2, Hannah E. Augustijn 3 4, Steven Huang 5, Haoqing Chen 1, Mary E. DeFeo 1 6, Kazuma Sekiba 1, Bi-Huei Hou 1, Xiandong Meng 7, Allison M. Weakley 7, Ashley V. Cabrera 7, Zhiwei Zhou 1, Gilles van Wezel 4 8, Marnix H. Medema 3 4, Calyani Ganesan 9, Alan C. Pao 9 10 11, Saurabh Gombar 1 2, Dylan Dodd 1
Department of Pathology, Stanford University School of Medicine, Stanford, CA 94305, USA
Atropos Health, Palo Alto, CA, USA
Bioinformatics Group, Wageningen University, Wageningen, the Netherlands
Institute of Biology, Leiden University, Leiden, the Netherlands
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720, USA
Department of Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA 94305, USA
ChEM-H Institute, Stanford University, Stanford, CA 94305, USA
Netherlands Institute of Ecology, Wageningen, the Netherlands
Division of Nephrology, Department of Medicine, Stanford University School of Medicine, Stanford, CA 94305, USA
Department of Urology, Stanford University School of Medicine, Stanford, CA 94305, USA
Veterans Affairs Palo Alto Health Care System, Palo Alto, CA 94304, USA
Read the paper in Cell: https://authors.elsevier.com/c/1hW-hL7PXmd2S
Key highlights, as summarized in earlier reports:
The choice of antibiotic may affect your gut microbiota which in turn influences uric acid metabolism and risk of gout. Atropos Health’s RWE capabilities supported the novel discovery of a widely distributed gene cluster integral to uric acid metabolism, and how uric acid metabolism may be influenced by antibiotics targeting different types of gut bacteria.
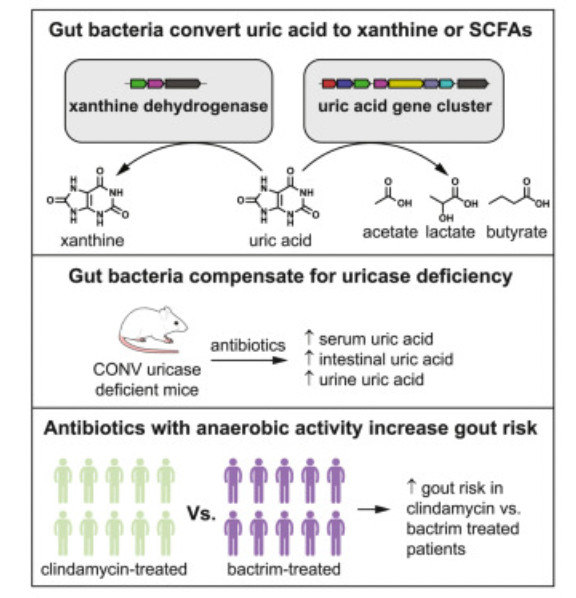
The authors sought to understand whether aerobic vs anaerobic antibiotics might exacerbate the risk of hyperuricemia and gout. These are prevalent conditions affecting ~15% and ~4% of US adults, respectively.
The authors hypothesized that patients on an anaerobic antibiotic (Clindamycin) would be at greater risk of developing gout compared to those on an aerobic antibiotic (Bactrim), since it targets anaerobic gut microbes involved in degrading uric acid and preventing hyperuricemia.
Atropos Health was engaged to conduct a retrospective cohort study using de-identified EHR data from Stanford Health Care and Atropos Evidence Platform’s proprietary technologies - such as the Advanced Cohorting Engine (ACE) and automated causal inference methods powered by high dimensional propensity scores.
Atropos leveraged these tools to compared new and recurrent gout diagnoses between new adult users of oral Clindamycin vs. oral Bactrim. A higher risk of gout for Clindamycin-treated patients was found after propensity-score matching (hazard ratio=1.3, 95% CI, 1.1-1.54), which also yielded 2 treatment groups similar in baseline characteristics such as demography, sex distribution, and comorbidities.
These findings suggest that “disruption of the anaerobic gut microbiota by antibiotics increases the risk of developing gout in human patients,” and support similar findings in uricase-knockout mice more sensitive to gut disruption.
Additionally, the mouse model demonstrated that uric acid metabolism in the gut might influence plasma levels in the host. Using isotope tracing and single knockout mutants of uric acid-inducible genes, the authors showed that gut bacteria with uric acid-inducible genes were critical for anaerobic uric acid metabolism. By identifying and characterizing these uric acid-inducible genes, the authors proposed a novel anaerobic mechanism for eliminating uric acid by gut bacteria.
Implications:
Antibiotic therapies that might disrupt the gut microbiota should be carefully considered in patients predisposed to gout
Increasing uric acid metabolism in the gut represents an important new therapeutic approach to treating patients with hyperuricemia
We envision that live biotherapeutic products consisting of uric acid consuming bacteria could be a new therapeutic modality to address outstanding needs in hyperuricemia and gout. Atropos Health’s contributions to this publication are consistent with our commitment to leveraging our rapid, consistent real-world evidence generation capabilities to advance research and the practice of evidence-based medicine. Make it stand out
To learn more about conducting research directly through Atropos Evidence Platform or joining an Atropos Research Collaborative, contact info@atroposhealth.com